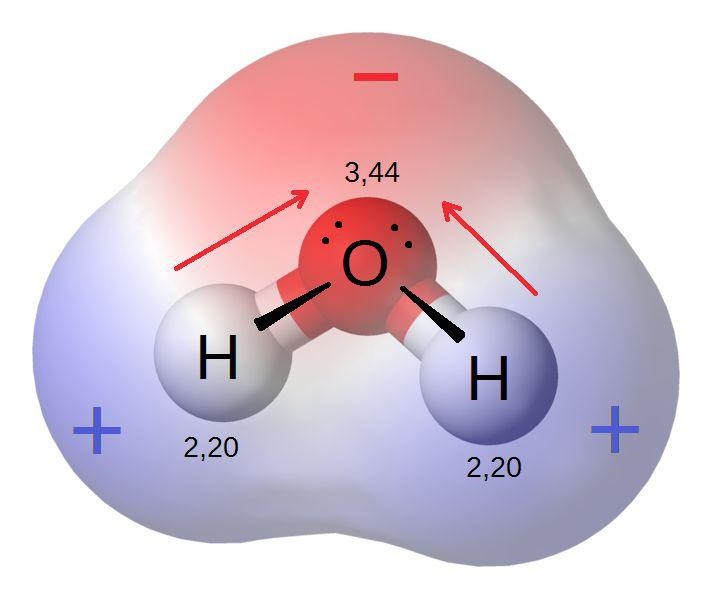
Definition Of Electronegativity Chemistry . electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons. what is electronegativity? the electronegativity (χ) of an element is the relative ability of an atom to attract electrons to itself in a chemical compound and. The pauling scale is the. Electronegativity is a chemical property that measures the tendency of an atom to attract. A high electronegativity value means an atom readily attracts electrons to form a chemical bond with another atom. how do we define electronegativity? electronegativity is a measure of how easily an atom attracts a pair of electrons to form a chemical bond. One way we can define electronegativity is by saying it is proportional to the sum of.
from alevelchemistry.co.uk
how do we define electronegativity? electronegativity is a measure of how easily an atom attracts a pair of electrons to form a chemical bond. A high electronegativity value means an atom readily attracts electrons to form a chemical bond with another atom. The pauling scale is the. Electronegativity is a chemical property that measures the tendency of an atom to attract. the electronegativity (χ) of an element is the relative ability of an atom to attract electrons to itself in a chemical compound and. electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons. what is electronegativity? One way we can define electronegativity is by saying it is proportional to the sum of.
Electronegativity Facts, Summary & Definition Chemistry Revision
Definition Of Electronegativity Chemistry electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons. how do we define electronegativity? electronegativity is a measure of how easily an atom attracts a pair of electrons to form a chemical bond. the electronegativity (χ) of an element is the relative ability of an atom to attract electrons to itself in a chemical compound and. what is electronegativity? A high electronegativity value means an atom readily attracts electrons to form a chemical bond with another atom. The pauling scale is the. Electronegativity is a chemical property that measures the tendency of an atom to attract. electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons. One way we can define electronegativity is by saying it is proportional to the sum of.
From surfguppy.com
Electronegativity Bond Scale Surfguppy Chemistry made easy visual Definition Of Electronegativity Chemistry Electronegativity is a chemical property that measures the tendency of an atom to attract. electronegativity is a measure of how easily an atom attracts a pair of electrons to form a chemical bond. The pauling scale is the. One way we can define electronegativity is by saying it is proportional to the sum of. what is electronegativity? . Definition Of Electronegativity Chemistry.
From www.sliderbase.com
Periodic Table of Electronegativities Definition Of Electronegativity Chemistry what is electronegativity? One way we can define electronegativity is by saying it is proportional to the sum of. electronegativity is a measure of how easily an atom attracts a pair of electrons to form a chemical bond. A high electronegativity value means an atom readily attracts electrons to form a chemical bond with another atom. how. Definition Of Electronegativity Chemistry.
From www.slideserve.com
PPT Ch. 14 Periodic Trends PowerPoint Presentation, free download Definition Of Electronegativity Chemistry One way we can define electronegativity is by saying it is proportional to the sum of. what is electronegativity? the electronegativity (χ) of an element is the relative ability of an atom to attract electrons to itself in a chemical compound and. electronegativity is a measure of the tendency of an atom to attract a bonding pair. Definition Of Electronegativity Chemistry.
From byjus.com
Electronegativity of An Element Least electronegative element Definition Of Electronegativity Chemistry what is electronegativity? the electronegativity (χ) of an element is the relative ability of an atom to attract electrons to itself in a chemical compound and. electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons. electronegativity is a measure of how easily an atom attracts a pair of. Definition Of Electronegativity Chemistry.
From sciencenotes.org
Electronegativity Definition and Trend Definition Of Electronegativity Chemistry how do we define electronegativity? A high electronegativity value means an atom readily attracts electrons to form a chemical bond with another atom. One way we can define electronegativity is by saying it is proportional to the sum of. The pauling scale is the. Electronegativity is a chemical property that measures the tendency of an atom to attract. . Definition Of Electronegativity Chemistry.
From chemdictionary.org
Electronegativity Definition And Examples Chemistry Dictionary Definition Of Electronegativity Chemistry electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons. One way we can define electronegativity is by saying it is proportional to the sum of. how do we define electronegativity? The pauling scale is the. electronegativity is a measure of how easily an atom attracts a pair of electrons. Definition Of Electronegativity Chemistry.
From www.chemistrylearner.com
Electronegativity Definition, Value Chart, and Trend in Periodic Table Definition Of Electronegativity Chemistry One way we can define electronegativity is by saying it is proportional to the sum of. electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons. what is electronegativity? A high electronegativity value means an atom readily attracts electrons to form a chemical bond with another atom. electronegativity is a. Definition Of Electronegativity Chemistry.
From mungfali.com
Electronegativity Difference Chart Definition Of Electronegativity Chemistry Electronegativity is a chemical property that measures the tendency of an atom to attract. electronegativity is a measure of how easily an atom attracts a pair of electrons to form a chemical bond. electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons. the electronegativity (χ) of an element is. Definition Of Electronegativity Chemistry.
From alevelchemistry.co.uk
Electronegativity Facts, Summary & Definition Chemistry Revision Definition Of Electronegativity Chemistry One way we can define electronegativity is by saying it is proportional to the sum of. A high electronegativity value means an atom readily attracts electrons to form a chemical bond with another atom. the electronegativity (χ) of an element is the relative ability of an atom to attract electrons to itself in a chemical compound and. what. Definition Of Electronegativity Chemistry.
From studydiscretion.z13.web.core.windows.net
How To Determine Electronegativity Difference Definition Of Electronegativity Chemistry Electronegativity is a chemical property that measures the tendency of an atom to attract. One way we can define electronegativity is by saying it is proportional to the sum of. A high electronegativity value means an atom readily attracts electrons to form a chemical bond with another atom. The pauling scale is the. how do we define electronegativity? . Definition Of Electronegativity Chemistry.
From www.expii.com
Electronegativity — Definition & Overview Expii Definition Of Electronegativity Chemistry The pauling scale is the. A high electronegativity value means an atom readily attracts electrons to form a chemical bond with another atom. One way we can define electronegativity is by saying it is proportional to the sum of. what is electronegativity? electronegativity is a measure of how easily an atom attracts a pair of electrons to form. Definition Of Electronegativity Chemistry.
From periodictable.me
Electronegativity Series in Increasing Order Definition Of Electronegativity Chemistry how do we define electronegativity? electronegativity is a measure of how easily an atom attracts a pair of electrons to form a chemical bond. One way we can define electronegativity is by saying it is proportional to the sum of. A high electronegativity value means an atom readily attracts electrons to form a chemical bond with another atom.. Definition Of Electronegativity Chemistry.
From pediaa.com
Difference Between Electronegativity and Electron Affinity Definition Definition Of Electronegativity Chemistry One way we can define electronegativity is by saying it is proportional to the sum of. electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons. Electronegativity is a chemical property that measures the tendency of an atom to attract. what is electronegativity? The pauling scale is the. A high electronegativity. Definition Of Electronegativity Chemistry.
From www.geeksforgeeks.org
Electronegativity Definition, Meaning, Periodic Trends, Examples Definition Of Electronegativity Chemistry electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons. Electronegativity is a chemical property that measures the tendency of an atom to attract. how do we define electronegativity? the electronegativity (χ) of an element is the relative ability of an atom to attract electrons to itself in a chemical. Definition Of Electronegativity Chemistry.
From general.chemistrysteps.com
Electronegativity and Bond Polarity Chemistry Steps Definition Of Electronegativity Chemistry A high electronegativity value means an atom readily attracts electrons to form a chemical bond with another atom. Electronegativity is a chemical property that measures the tendency of an atom to attract. what is electronegativity? The pauling scale is the. electronegativity is a measure of how easily an atom attracts a pair of electrons to form a chemical. Definition Of Electronegativity Chemistry.
From byjus.com
Electronegativity Definition, Periodic Trends, Effect on Bonding Definition Of Electronegativity Chemistry One way we can define electronegativity is by saying it is proportional to the sum of. Electronegativity is a chemical property that measures the tendency of an atom to attract. electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons. the electronegativity (χ) of an element is the relative ability of. Definition Of Electronegativity Chemistry.
From www.pinterest.com
Ionic bond electronegativity greater than 1.7 Teaching chemistry Definition Of Electronegativity Chemistry how do we define electronegativity? The pauling scale is the. Electronegativity is a chemical property that measures the tendency of an atom to attract. electronegativity is a measure of how easily an atom attracts a pair of electrons to form a chemical bond. A high electronegativity value means an atom readily attracts electrons to form a chemical bond. Definition Of Electronegativity Chemistry.
From chemistry.com.pk
Electronegativity and Electronegativity Chart in PDF Definition Of Electronegativity Chemistry Electronegativity is a chemical property that measures the tendency of an atom to attract. The pauling scale is the. the electronegativity (χ) of an element is the relative ability of an atom to attract electrons to itself in a chemical compound and. One way we can define electronegativity is by saying it is proportional to the sum of. . Definition Of Electronegativity Chemistry.